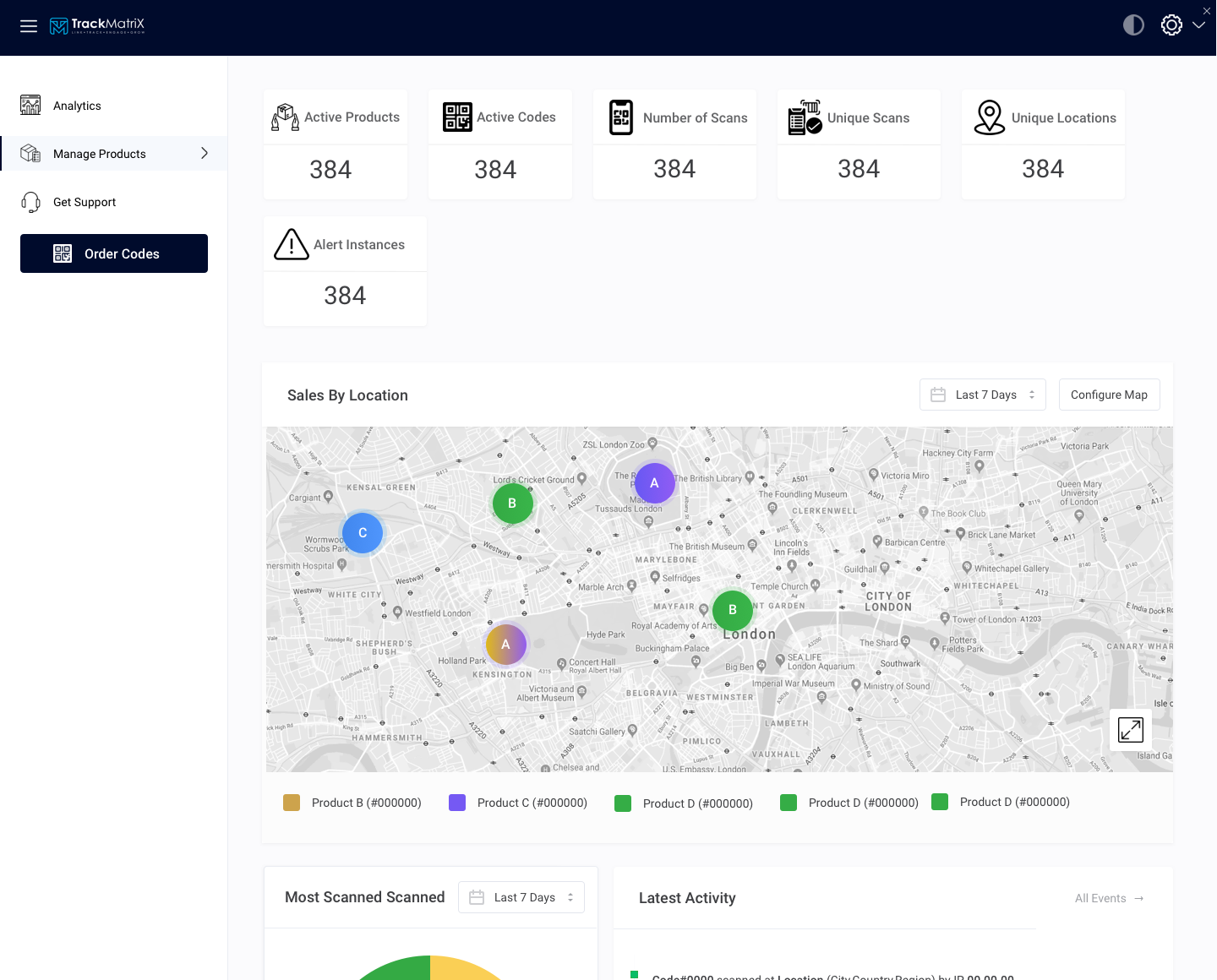
Pharma Track and Trace Serialization Software
Pharmaceutical counterfeiting can be as small as one person with a printer and barcode scanner mislabeling medications and as large as a criminal organization that stretches across multiple countries. This is why pharma companies need to have an effective and comprehensive solution in order to combat fake medications.
Government regulation is also catching up as the US FDA’s Drug Supply Chain Security Act (Title II of the Drug Quality and Security Act) requires product tracing (electronic and interoperable system) at the package-level by 2023.